URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: New technique enables spatial separation of peptide structures
News
News from the DESY research centre
New technique enables spatial separation of peptide structures
A team of scientists at DESY and Universität Hamburg has reached another milestone towards the direct imaging of individual biomolecules: the group led by Jochen Küpper from the Center for Free-Electron Laser Science developed a new experimental technique which enables the separation of different peptide structures in order to analyze and image them separately. The scientists report their method, which ultimately can be applied in diverse experiments, in the scientific journal Angewandte Chemie International Edition.
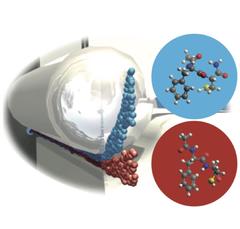
A current method to study such interactions in detail is to study isolated small peptides, that is chains of single amino acids, in the gas phase. However, even single amino acids and small peptides can arrange themselves into different 3-dimensional structures, so called conformers. This fact makes a detailed analysis of these important biomolecular building blocks rather complicated, since techniques such as X-ray diffraction require identical 3-dimensional structures to produce structural data at atomic resolution.
“Our aim therefore was to develop new experimental techniques that produce peptide samples in the gas phase with identical 3-dimensional structures,” says Nicole Teschmit from the cluster of excellence CUI (Centre for Ultrafast Imaging) at Universität Hamburg, first author of the study. The team used laser desorption to produce very cold molecular beams of intact dipeptide molecules, which were then identified by laser spectroscopy. At minus 271 degrees Celsius, the different conformers no longer interconvert in such a cold molecular beam. To spatially separate the different structures, the scientists used strong electric fields that interact with the specific dipole moments of the different conformers and deflect them to different extends. With this method the scientists now succeeded in completely spatially separating the two conformers of the prototypical dipeptide Ac-Phe-Cys-NH2 and producing pure samples of either conformer in the gas phase.
“We succeeded for the first time in demonstrating cold molecular beams of conformer-selected peptides. Such samples will enable the analysis of conformer-specific processes with general techniques which usually cannot differentiate between structures,” co-author Daniel Horke says. Furthermore, the low temperatures of the generated molecular ensembles allow for strongly fixing the molecules in space. This is a prerequisite for the recording of atomically resolved images of biomolecules, as Küpper points out: “Our method is a milestone on the road toward a direct structural imaging of biological molecules.”
Reference:
Spatially separating the conformers of the dipeptide Ac-Phe-Cys-NH2; Nicole Teschmit, Daniel A. Horke, Jochen Küpper; Angewandte Chemie International Edition, 2018; DOI: 10.1002/anie.201807646



