URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: Water is not simply water
News
News from the DESY research centre
Water is not simply water
Water molecules exist in two different forms with almost identical physical properties. For the first time, researchers have now shown that these forms can exhibit different chemical reactivities. These results were reported by researchers from the University of Basel and their colleagues from DESY and the University of Hamburg in the scientific journal Nature Communications.
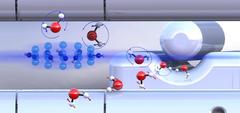
Pre-sorted ortho-water and para-water molecules with differently oriented nuclear spins (blue or red arrows) react with diazenylium ions (center left) at different speeds. Credit: Ardita Kilaj, University of Basel
The research group headed by Professor Stefan Willitsch from the University of Basel’s Department of Chemistry has investigated how the two forms of water differ in terms of their chemical reactivity – their ability to undergo a chemical reaction. To test this, the two isomers have to be separated. “Para- and ortho-water have almost identical physical properties which makes their separation particularly challenging,” explains Ardita Kilaj from the University of Basel, first author of the paper. “Also, the molecules frequently collide with other molecules, causing nuclear spin orientations to change so that para- and ortho-water transform into one another,” adds co-author Professor Jochen Küpper from DESY and the University of Hamburg.
The separation of the two forms of water was made possible by an “electric prism” developed by Küpper's group at the Center for Free-Electron Laser Science CFEL at DESY: The scientists send an extremely thin jet of water molecules through a strong electric field. “Para- and ortho-water get deflected differently, allowing us to separate them in space and obtain nearly pure para and ortho samples,” explains Küpper, who is also a member of the Hamburg Centre for Ultrafast Imaging CUI.
Using this approach, the researchers were able to initiate controlled reactions between the sorted water isomers and ultracold diazenylium ions (“protonated nitrogen”) held in a trap. During this process, a diazenylium ion transfers its proton to a water molecule. This reaction is also observed in the chemistry of interstellar space.
“It was demonstrated that para-water reacts about 25 per cent faster than ortho-water,” says Willitsch, who led the research. “This effect can be explained in terms of the nuclear spin also influencing the rotation of the water molecules. As a result, different attractive forces act between the reaction partners. Para-water is able to attract its reaction partner more strongly than the ortho-form, which leads to an increased chemical reactivity.” Computer simulations confirmed these experimental findings.
In their experiments, the researchers worked with molecules at very low temperatures close to absolute zero (about –273 degrees Celsius). These are ideal conditions to precisely prepare individual quantum states and define the energy content of the molecules, and to cause a controlled reaction between them. Willitsch explains the experimental approach: “The better one can control the states of the molecules involved in a chemical reaction, the better the underlying mechanisms and dynamics of a reaction can be investigated and understood.”
Reference:
Observation of different reactivities of para- and ortho-water towards trapped diazenylium ions; Ardita Kilaj, Hong Gao, Daniel Rösch, Uxia Rivero, Jochen Küpper, Stefan Willitsch; Nature Communications, 2018; DOI: 10.1038/s41467-018-04483-3
Further reading:
- Press release of the University of Basel
- „Behind the paper“ blog post
- The study has been chosen as a Highlight in Inorganic and Physical Chemsitry by “Nature Communications”



