URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: Crystals in a pink X-ray beam
News
News from the DESY research centre
Crystals in a pink X-ray beam
A newly developed experimental set-up allows the X-ray structure determination of biomolecules such as proteins with far smaller samples and shorter exposure times than before. At so-called synchrotron sources, protein crystal can be studied considerably more efficiently and quickly by using broad-spectrum X-rays. However, due to the large amount of scattered radiation, this has until now required very large crystals. The newly developed experimental setup now allows the unwanted scattered radiation to be substantially reduced, so that scientists have been able to perform serial crystallography using broad-spectrum synchrotron radiation for the first time. The international team led by DESY scientist Alke Meents published its findings from experiments at the Advanced Photon Source (APS) in the U.S. in the journal Nature Communications.
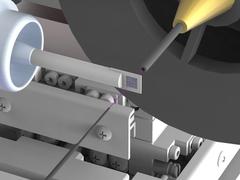
The newly developed set-up tremendously reduces background scattering, which hampers in particular high resolution structure determinations. To achieve this, the X-ray beam (highlighted in pink) is enclosed in thin metal capillaries shortly before and behind the sample and the air immediately surrounding the sample is replaced by a stream of helium gas. Credit: DESY, Julian Bergtholdt
In contrast to conventional X-ray crystallography, which is usually carried out on single or a small number of large protein crystals, serial crystallography looks at a hundred to hundreds of thousands of very small crystals. The information collected is then merged to form a data set from which the crystal structure can be deduced. This method has already been applied frequently using X-ray free-electron lasers (XFELs). In addition, by employing the very short X-ray pulses of XFELs, chemical and enzyme reactions can be studied over short time intervals.
“Serial crystallography can also be carried out easily using synchrotron sources. However, the measurements take longer with those sources, because they use monochromatic X-rays of a specific colour, resulting in a low flux of the X-rays,” explains Henry Chapman, leading scientist at DESY and co-author of the paper. At synchrotrons, usually just a narrow band of X-ray wavelengths is used for this kind of analysis, so that just a small fraction of the available X-ray photons is used for the experiment.. “Also, we need many crystals to collect a complete set of data – often several tens of thousands. Since the exposure times are relatively long using a synchrotron, the only way to study fast reactions has been with an X-ray laser,” says Chapman.

Until now, however, it has not been possible to carry out serial crystallography using the pink beam because the measurements are severely impaired by high levels of background produced with such strong beams. “Unwanted background in the measured patterns is produced because the X-rays used to probe the sample are not only scattered by the molecules in the crystal itself, but also by the sample holder and the surrounding air,” explains Max Wiedorn, DESY co-author of the paper . “The actual signal measured during experiments with the pink beam is spread out over the many “colours”, meaning that the scattered background radiation has a distinctly more negative impact on the measurements than is the case using monochromatic radiation.”
The scientists have therefore come up with a new set-up, which largely suppresses the unwanted scattered radiation. They use a sample holder made of silicon, which does not scatter X-rays; also, they ensure that there is very little air in the path of the X-ray beam. To achieve this, the X-ray beam is enclosed in a thin metal tube right before and behind the sample, which prevents the scattered radiation from reaching the X-ray camera. Also, the air immediately surrounding the sample is replaced with a stream of helium gas, which causes less scattering than air. By suppressing scattered radiation, the scientists have now managed for the first time to determine the three-dimensional structure of two proteins to a very high precision by means of pink beam serial crystallography at a synchrotron. The corresponding measurements were made at the BioCARS beamline of the Advanced Photon Source (APS) at the Argonne National Laboratory in the United States.
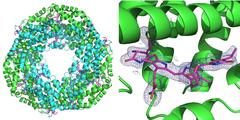
Left: The first measurement carried out using the new set-up was performed on the protein phycocyanin that plays a role for photosynthesis in cyanobacteria. It forms a ring of six double molecules (heterodimers). At each heterodimer three phycocyanobilin molecules (pink) can bind. Right: The blue grid represents the electron density of a bound phycocyanobilin molecule. Both structures were calculated from just 52 diffraction images recorded with the pink beam of the Advanced Photon Source (APS) at the BioCARS beamline. Credit: DESY, Julia Lieske
Furthermore, because measurements can be performed at room temperature, structural changes such as those that occur when an active substance binds to a targeted protein can in future be studied over time. The new method achieves a very high temporal resolution of 100 picoseconds, something that was not possible at synchrotrons with the monochromatic X-rays used in the past. A picosecond is a millionth of a millionth of a second. A beam of light travels about three centimetres in 100 picoseconds.
Scientists from the University of Hamburg, the University of Chicago, the Medical University of Hannover, Argonne National Laboratory and the National Science Foundation, BioXFEL Science and Technology Center, in the US were also involved in the research.
Reference: Pink-beam serial crystallography; A. Meents, M.O. Wiedorn, V. Srajer, R. Henning, I. Sarrou, J. Bergtholdt, M. Barthelmess, P.Y.A. Reinke, D. Dierksmeyer, A. Tolstikova, S. Schaible, M. Messerschmidt, C.M. Ogata, D.J. Kissick, M.H. Taft, D.J. Manstein, J. Lieske, D. Oberthuer, R.F. Fischetti, H.N. Chapman; „Nature Communications“, 2017; DOI: 10.1038/s41467-017-01417-3