URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: Under pressure: Heliums forms stable compound
News
News from the DESY research centre
Under pressure: Heliums forms stable compound
Using high pressure, an international team of researchers has produced a stable helium compound. The discovery comes as a surprise as Helium does not react with other elements under normal conditions. The scientists around Artem R. Oganov of the Skolkovo Institute of Science and Technology (Skoltech) in Russia, Stony Brook University in the USA and the Moscow Institute of Physics and Technology report in the journal Nature Chemistry how they predict two stable compounds of helium and sodium and were able to confirm one of them in experiments partly carried out at DESY.
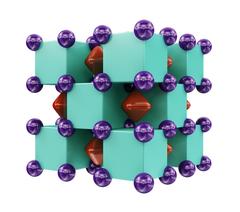
The scientist were able to synthesise Na2He using a so-called diamond anvil cell that produces very high pressures. The compound appeared at pressures of about 1.1 million times Earth’s atmospheric pressure and is predicted to be stable at least up to 10 million times that. Evidence of the compound was found using X-ray diffraction experiments that were also carried out at the Extreme Conditions Beamline P02.2 at DESY's high-intensity X-ray source PETRA III. At the Extreme Conditions Beamline, material samples can be analysed at extremely high pressures and temperatures. DESY scientist Zuzana Konôpková was involved in the experiments as a co-author of the study.
“The compound that we discovered is very peculiar: helium atoms do not actually form any chemical bonds, yet their presence fundamentally changes chemical interactions between sodium atoms, forces electrons to localize inside cubic voids of the structure and makes this material insulating,” says Xiao Dong, the first author of this work. Na₂He is what’s called an electride, which is a special type of an ionic salt-like crystal. It has a positively charged sublattice of sodium ions and another negatively charged sublattice formed of localized electron pairs.
The second compound, Na2HeO, is predicted to be stable in the pressure range from 0.15 to 1.1 million atmospheres. It is also an ionic crystal with a structure similar to that of Na₂He. However, in place of electron pairs, it has negatively charged oxygen in the form of O²⁻.
“This study shows how new surprising phenomena can be discovered by combination of powerful theoretical methods and state-of-the-art experiments,” says Oganov. The results could further our understanding of the chemistry occurring inside gas giant planets and possibly even stars, where helium is a major component.
Reference:
A stable compound of helium and sodium at high pressure; Xiao Dong, Artem R. Oganov, Alexander F. Goncharov, Elissaios Stavrou,
Sergey Lobanov, Gabriele Saleh, Guang-Rui Qian, Qiang Zhu, Carlo Gatti, Volker L. Deringer, Richard Dronskowski, Xiang-Feng Zhou, Vitali Prakapenka, Zuzana Konôpková, Ivan Popov, Alexander I. Boldyrev and Hui-Tian Wang; „Nature Chemistry“, 2017; DOI: 10.1038/NCHEM.2716



